Clinical trial data shows that the active ingredient in EnteraGam significantly reduces the number of days you may experience bloating, diarrhea, and flatulence.
Intended Use
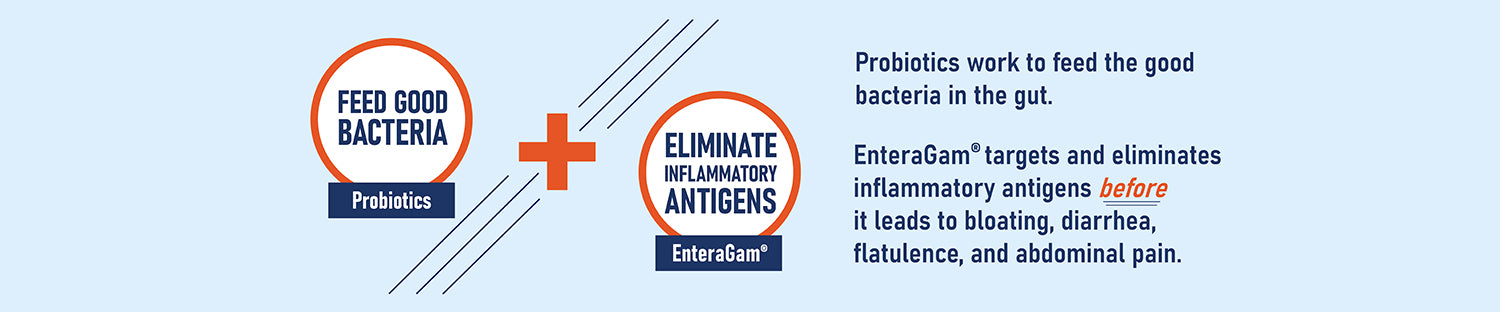
EnteraGam® is a medical food product intended for the dietary management of chronic diarrhea and loose stools. EnteraGam® must be administered under medical supervision.
Important Safety Information
EnteraGam® contains beef protein: therefore, patients who have an allergy to beef or any other component of EnteraGam® should not take this product. EnteraGam® has not been studied in pregnant women, in women during labor and delivery, or in nursing mothers. The choice to administer EnteraGam® during pregnancy, labor and delivery, or to nursing mothers is at the clinical discretion of the healthcare provider.
EnteraGam® does not contain any milk-derived ingredients such as lactose, casein, or whey. EnteraGam® is gluten-free, dye-free, and soy-free.
To report suspected adverse reactions, contact Entera Health, LLC at 1-855-4ENTERA (1-855-436-8372), or the FDA at 1-800 FDA-1088 (1-800-332-1088) or www.fda.gov/medwatch.